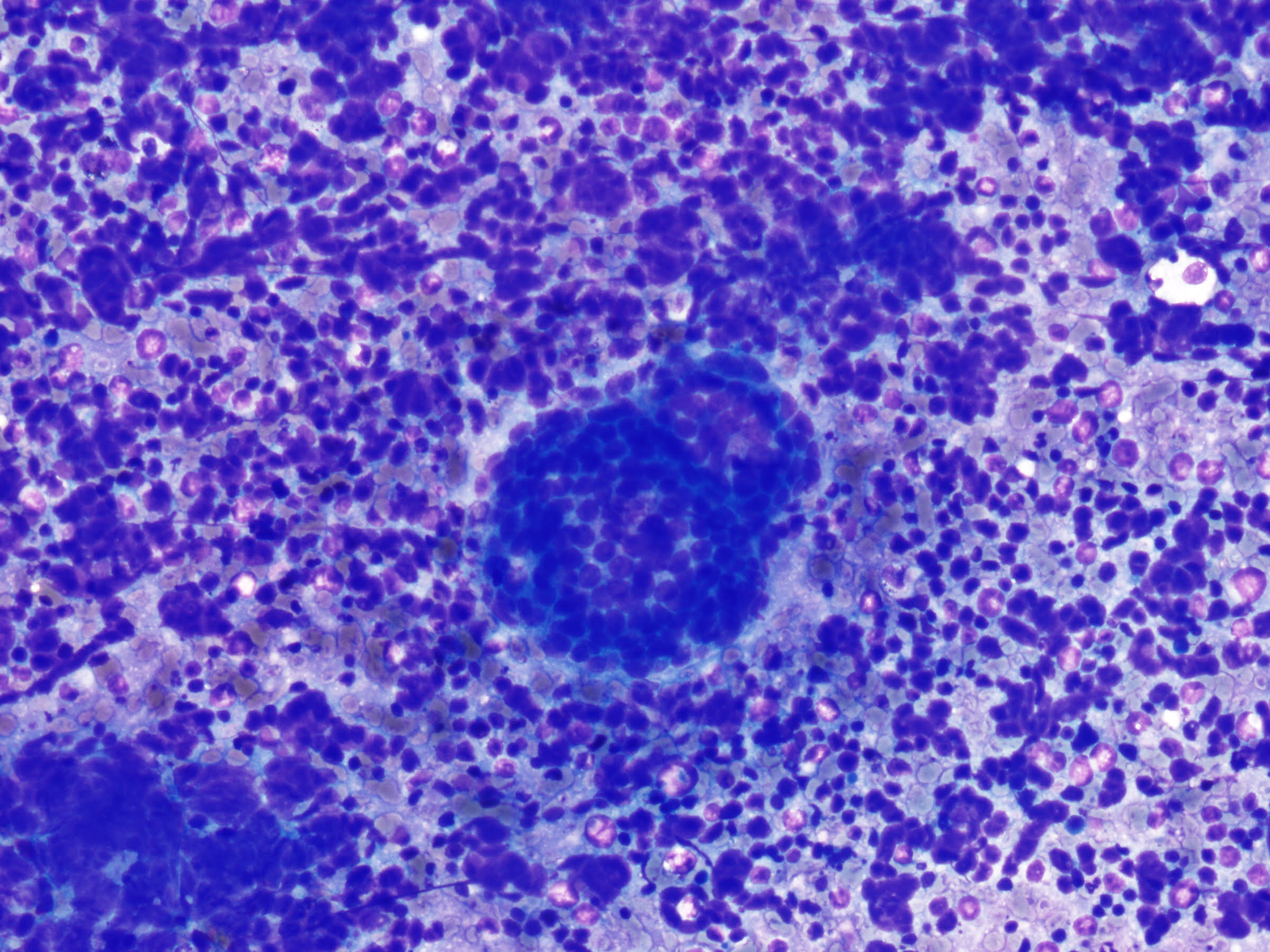
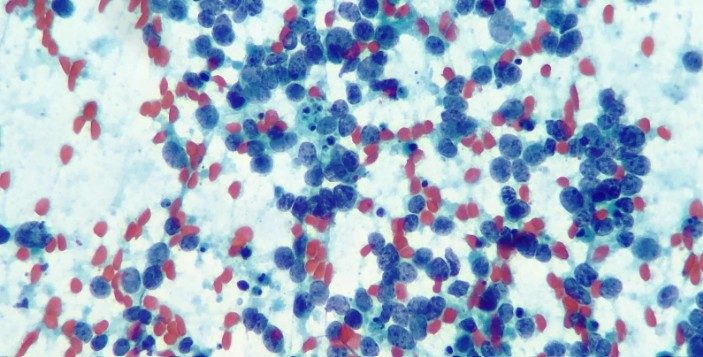
Case of the Month May by Cisel Aydin
COMBINED SMALL CELL CARCINOMA AND ADENOCARCINOMA
DIAGNOSED ON EBUS-TBNA MATERIAL
Author: Cisel Aydin Mericoz MD, Pinar Firat MD,
Institution: Koç University Hospital, Department of Pathology, İstanbul, Türkiye
CLINICAL PRESENTATION:
A 64-year-old male patient presented with a 2.5-month history of numbness and severe pain in the arms and legs. He was initially treated with physical therapy, without clinical improvement. A thoracic CT scan revealed a 2 cm, well-circumscribed lesion in the left upper lobe and extensive mediastinal lymphadenopathy, including the left hilar region. EBUS-guided transbronchial needle aspiration (EBUS-TBNA) was performed for diagnostic purposes.